Abstract
Introduction
Cocoa flavanols (CF) may exert health benefits through their potent vasodilatory effects, which are perpetuated by elevations in nitric oxide (NO) bioavailability. These vasodilatory effects may contribute to improved delivery of blood and oxygen (O2) to exercising muscle.
Purpose
Therefore, the objective of this study was to examine how CF supplementation impacts pulmonary O2 uptake (\(\dot{V}{\text{O}}_{2}\)) kinetics and exercise tolerance in sedentary middle-aged adults.
Methods
We employed a double-blind cross-over, placebo-controlled design whereby 17 participants (11 male, 6 female; mean ± SD, 45 ± 6 years) randomly received either 7 days of daily CF (400 mg) or placebo (PL) supplementation. On day 7, participants completed a series of ‘step’ moderate- and severe-intensity exercise tests for the determination of \(\dot{V}{\text{O}}_{2}\) kinetics.
Results
During moderate-intensity exercise, the time constant of the phase II \(\dot{V}{\text{O}}_{2}\) kinetics (\(\tau \dot{V}{\text{O}}_{2}\)) was decreased by 15% in CF as compared to PL (mean ± SD; PL 40 ± 12 s vs. CF 34 ± 9 s, P = 0.019), with no differences in the amplitude of \(\dot{V}{\text{O}}_{2}\) (A\(\dot{V}{\text{O}}_{2}\); PL 0.77 ± 0.32 l min−1 vs. CF 0.79 ± 0.34 l min−1, P = 0.263). However, during severe-intensity exercise, \(\tau \dot{V}{\text{O}}_{2}\), the amplitude of the slow component (\({\text{SC}}\dot{V}{\text{O}}_{2}\)) and exercise tolerance (PL 435 ± 58 s vs. CF 424 ± 47 s, P = 0.480) were unchanged between conditions.
Conclusion
Our data show that acute CF supplementation enhanced \(\dot{V}{\text{O}}_{2}\) kinetics during moderate-, but not severe-intensity exercise in middle-aged participants. These novel effects of CFs, in this demographic, may contribute to improved tolerance of moderate-activity physical activities, which appear commonly present in daily life.
Trial registration
Registered under ClinicalTrials.gov Identifier no. NCT04370353, 30/04/20 retrospectively registered
Similar content being viewed by others
Introduction
Skeletal muscle \(\dot{V}{\text{O}}_{2}\) contraction and force production form the basis for the ability to perform physical activity, both for daily life activities as well as during sports-related events. Repeated muscle contractions require continuous regeneration of adenosine triphosphate (ATP). The production of ATP during (prolonged) physical activity is driven through the mechanism of oxidative phosphorylation, which depends on sufficient availability of oxygen (O2) amongst other key substrates (Poole et al. 2008). Impairment to pathways involved in the delivery of O2 to working skeletal muscle, like that observed in older and physically inactive adults, leads to slower rates of pulmonary O2 uptake (\(\dot{V}{\text{O}}_{2}\)) and therefore greater O2 deficit (Cunningham and Paterson 1994; DeLorey et al. 2004a; Dumanoir et al. 2010; Whipp and Rossiter 2013; George et al. 2018). Slower \(\dot{V}{\text{O}}_{2}\) kinetics in response to physical activity are associated with lower exercise tolerance (Grassi et al. 2011; Goulding et al. 2017, 2018), and may affect the capacity to perform daily life activities that require moderate-intensity physical activity.
The slower dynamic adjustment of \(\dot{V}{\text{O}}_{2}\) across a metabolic transient observed in older adults is thought to be due to a mismatch of O2 delivery to O2 utilisation (Murias et al. 2010; Murias and Paterson 2015; George et al. 2018). Indeed, attenuations in microvascular blood flow supply and distribution (and thus O2 delivery) within aged skeletal muscle are well documented (Muller-Delp et al. 2002; Musch et al. 2004; Behnke and Delp 2010; Dumanoir et al. 2010). These reductions in O2 delivery to active skeletal muscle are likely caused by impaired vascular endothelial function and diminished nitric oxide (NO) bioavailability (Muller-Delp et al. 2002; Woodman et al. 2002; Spier et al. 2004; Sindler et al. 2009). Interestingly, lifestyle interventions, such as exercise training and dietary strategies (Vanhatalo et al. 2010; Schreuder et al. 2015), have demonstrated potent effects to enhance NO bioavailability and improve endothelial function. Consequently, a number of studies have shown faster \(\dot{V}{\text{O}}_{2}\) kinetics in concert with increased O2 availability (Murias et al. 2010; Bailey et al. 2015; Goulding et al. 2017).
Cocoa flavanols (CFs) represent a group of flavonoids present in cocoa derived from seeds of the fruit of the Theobroma cacao tree. Previous studies have found CFs (700–900 mg range) act primarily through the monomer (−)-epicatechin, to stimulate NO production, resulting in improved vasodilation and endothelial function in healthy adults (Schroeter et al. 2006; Davison et al. 2008; Berry et al. 2010). Given the direct impact of CFs on NO production and vascular endothelial function, and the negative effects of sedentary ageing on O2 delivery and \(\dot{V}{\text{O}}_{2}\) kinetics at the onset of exercise, our objective was to test the hypothesis that, compared with placebo (PL), CF supplementation speeds phase II \(\dot{V}{\text{O}}_{2}\) kinetics during moderate-intensity physical activity and increases exercise tolerance in healthy middle-aged individuals.
Methodology
Participants
Seventeen healthy middle-aged adults (11 male: mean ± SD, age 45 ± 6 years; body mass 87.7 ± 13.5 kg; height 1.75 ± 0.07 m; and 6 female: aged 47 ± 5 years; body mass 68.2 ± 17.7 kg; height 1.62 ± 0.09 m) volunteered and gave written informed consent to participate in the study (see Fig. 1). All procedures conformed to the Declaration of Helsinki and were approved by Liverpool John Moores University Research Ethics Committee (approval reference number: 18/SPS/014). Participants engaged in less than two hours of structured exercise training per week. All participants were non-smokers and had no history of cardiovascular, respiratory or metabolic diseases. Participants were not taking any dietary supplements or medication.
Participants reported to the laboratory at least 3 h postprandial in a rested state, having completed no strenuous exercise within the previous 24 h and avoided alcohol and caffeine for 24 and 6 h, preceding each exercise test, respectively. Participants were advised to avoid consumption of flavonoid-rich foodstuffs (e.g. green tea, dark chocolate and berries) in the 24 h preceding each experimental trial.
Procedures
Participants visited the temperature-controlled laboratory (19–22 °C) on four occasions during a 4–5-week period, with each test scheduled at the same time of day (± 1 h) and at least 48 h between visits. Participants completed two preliminary trials and two experimental trials. Exercise bouts were performed on an electrically operated cycle ergometer (Lode Corival, Groningen, The Netherlands). Saddle and handlebar height/angle were recorded at the first visit and replicated during each subsequent visit for each individual participant. Throughout all exercise tests, participants were instructed to maintain a cadence of 65–80 rev min−1, and exhaustion was defined as when the participants cadence dropped 10 rev min−1 below the target work rate. Time to exhaustion was measured to the nearest second (s) in all tests.
Preliminary trial(s)
Upon arrival to the laboratory, participants’ height and weight were recorded. Subsequently, each participant undertook an incremental step test until the limit of tolerance to establish \(\dot{V}{\text{O}}_{2}\) peak, the gas exchange threshold (GET) and the power outputs for later tests. The incremental step test consisted of 3-min of baseline pedalling at 0 W, followed by a continuous, stepped increase in power output of 30 or 25 W every minute (for males and females, respectively) until the limit of tolerance was established. Gas exchange and ventilatory variables were measured continuously at the mouth breath-by-breath throughout each test. \(\dot{V}{\text{O}}_{2}\) peak was defined as the highest \(\dot{V}{\text{O}}_{2}\) value obtained over 30 s. The GET was determined using a collection of previously established criteria (Beaver et al. 2016) including (1) a disproportionate rise in CO2 production (\(\dot{V}{\text{CO}}_{2}\)) relative to \(\dot{V}{\text{O}}_{2}\); (2) an increase in minute ventilation (\(\dot{V}{\text{E}}\)) relative to \(\dot{V}{\text{O}}_{2}\) (\({{\dot{V}{\text{E}}} \mathord{\left/ {\vphantom {{\dot{V}{\text{E}}} {\dot{V}{\text{O}}_{2} }}} \right. \kern-\nulldelimiterspace} {\dot{V}{\text{O}}_{2} }}\)) without an increase in \(\dot{V}{\text{E}}\) relative to \(\dot{V}{\text{CO}}_{2}\) (\({{\dot{V}{\text{E}}} \mathord{\left/ {\vphantom {{\dot{V}{\text{E}}} {\dot{V}{\text{CO}}_{2} }}} \right. \kern-\nulldelimiterspace} {\dot{V}{\text{CO}}_{2} }}\)); and (3) an increase in end tidal O2 tension without decreasing end tidal CO2 tension.
During the familiarisation trial (visit 2), participants were requested to perform two bouts of severe-intensity exercise at a fixed power output to exhaustion (e.g. Tlim), each separated by 45 min of seated rest. The power outputs of these severe-intensity bouts were selected based upon performance during the incremental test and were calculated to be 60% ∆ (i.e., a work rate calculated to require 60% of the difference between GET and \(\dot{V}{\text{O}}_{2}\) peak). On occasion, adjustments were made to the power output of the subsequent exercise tests based upon performance in the familiarisation trials; the prescribed power output was lowered for participants who failed to exercise for up to 360 s during the severe-intensity bouts.
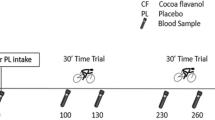
After completion of the familiarisation trial, participants were randomly assigned (computer generated), using a double-blind cross-over design (Fig. 2), to receive seven consecutive days of CF supplementation or a PL that was matched for caffeine and theobromine content. Nine participants began with the CF condition, and eight participants began with the PL condition. Participants were advised to consume four capsules daily. Each CF capsule contained 316 mg CocoActiv (Naturex, Netherlands; ~ 100 mg total flavanols of which 22 mg DP1 = catechin + epicatechin) whereas PL capsules contained 0 mg CocoActiv product. This CF dose was selected based on the knowledge that ~ 400 mg CF’s are required to improve vascular function during exercise (Decroix et al. 2018a). Both PL and CF capsules contained 2.9 mg caffeine and 22.5 mg theobromine (Fagron, Netherlands). Remaining empty volumes of PL and CF capsules were filled with microcrystalline cellulose (Fagron, Netherlands). Two capsules were taken in the morning and two in the evening following ingestion of a mixed meal (Cifuentes-Gomez et al. 2015). A 7-day wash-out period separated the supplementation periods and the order between CF and PL supplementation was randomised. Throughout the study period participants were instructed to maintain their normal daily activities and diet. Participants kept a food dairy and were instructed to consume an identical diet in the two periods of exercise testing. Physical activity levels were measured by accelerometery in the 6 days preceding testing via a hip-mounted activity monitor (Actigraph GT3X).
Experimental trials
On the 7th day of supplementation, participants were advised to consume four capsules 45 min prior to arrival at the laboratory. The supplementation protocol was chosen so that participants commenced exercise testing ~ 90 min following supplement ingestion, which coincided with reported peak plasma flavanol concentrations (Cifuentes-Gomez et al. 2015). The participants completed a series of separate “step” exercise tests from an unloaded (0 W) baseline to moderate or severe-intensity work rates for the determination of pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics. Tests began with 3 min of 0 W baseline cycling, before a step change in power output to 80% GET for 6 min or 60% ∆ until Tlim. Participants sequentially completed three bouts of moderate- and one bout of severe-intensity exercise, each separated by 10 min of passive recovery. This protocol was employed with the knowledge that multiple bouts of moderate-intensity exercise do not impact the \(\dot{V}{\text{O}}_{2}\) kinetics of subsequent moderate- and heavy-intensity exercise (Burnley et al. 2000; Spencer et al. 2011).
Measurements
After arrival to the laboratory, participants underwent an assessment of the previous 7 days physical activity levels and sedentary behaviour by the International Physical Activity Questionnaire (IPAQ) and by accelerometery (ActiGraph GTX3) (data not shown). Following 10 min of seated rest, participants blood pressure was measured in the brachial artery. Blood pressure was measured three times and the mean of the responses was recorded.
During all exercise tests, pulmonary gas exchange and ventilation were measured at the mouth breath-by-breath using a metabolic cart (Jaeger Oxycon Pro, Hoechberg, Germany). Participants wore a facemask and breathed through a low dead space (90 ml), low resistance (0.75 mmHg l−1 s−1 at 15 l/s) impeller turbine assembly (Jaeger Triple V, Hoechberg, Germany). The inspired and expired gas volumes and gas concentration signals were continuously sampled at 100 Hz, the latter using paramagnetic (O2) and infrared (CO2) analysers (Jaeger Oxycon Pro, Hoechberg, Germany) via a capillary line connected to the mouthpiece. These analysers were calibrated before each test with gases of known concentrations (16% O2 and 4% CO2), and the turbine volume transducer was calibrated using a 3-L syringe (Hans Rudolph, Kansas City, MO). The volume and concentration signals were time aligned by accounting for the delay in capillary gas transit and analyser rise time relative to the volume signal. Breath-by-breath fluctuations in lung gas stores were corrected for by computer algorithms (Beaver et al. 1981). Heart rate was measured during all tests via short-range radiotelemetry (Polar H10, Polar Electro, Kempele, Finland). During one of the transitions to moderate- and severe-intensity exercise for both supplementation periods, a blood sample was collected from a fingertip over the last 30 s preceding the step transition in work rate and within the last 15 s of exercise. Blood samples were immediately analysed using a hand-held device (Lactate Pro, Nova Biomedical, USA) to determine blood lactate concentration. Blood lactate accumulation was calculated as the difference between blood lactate at end exercise and blood lactate at baseline.
Data analysis
Breath-by-breath \(\dot{V}{\text{O}}_{2}\) data were edited to remove data points lying more than three standard deviations (SD) outside the local 5-breath mean (Lamarra et al. 1987). The resultant data were then linearly interpolated to provide second-by-second values. For \(\dot{V}{\text{O}}_{2}\) and heart rate data in response to moderate exercise transitions, second-by-second data for the three transitions were averaged together to produce a single dataset. The severe-intensity exercise bout for each condition was not repeated and was modelled separately. For each exercise transition, the first 20 s of data after the onset of exercise (i.e., the cardiodynamic or phase I response) was deleted (McNarry et al. 2012; Benson et al. 2017) and a mono-exponential model (Eq. 1) with time delay was then fitted to the data (Whipp and Rossiter 2013), as follows:
where \(\dot{V}{\text{O}}_{2}\)(t) is the \(\dot{V}{\text{O}}_{2}\) at any time t, \(\dot{V}{\text{O}}_{{2{\text{b}}}}\) is the baseline \(\dot{V}{\text{O}}_{2}\), which was taken as the mean \(\dot{V}{\text{O}}_{2}\) over the final 30 s of the baseline period preceding the transition, \(A\dot{V}{\text{O}}_{2}\)is the amplitude of the primary response above baseline, \({\text{TD}}_{{\dot{V}{\text{O}}_{2} }}\) is the time delay of the primary response relative to the onset of exercise, and \(\tau \dot{V}{\text{O}}_{2}\) is the time constant of the primary response. For moderate-intensity exercise, data were modelled to 360 s. For severe-intensity exercise, the onset of the \(\dot{V}{\text{O}}_{2}\)slow component (\({\text{TD}}_{{{\text{SC}}\dot{V}{\text{O}}_{2} }}\)) was determined using purpose-designed programming in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA), which iteratively fits a monoexponential function to the \(\dot{V}{\text{O}}_{2}\) data, starting at 60 s until the window encompasses the entire response. The resulting primary time constants are plotted against time, and the \({\text{TD}}_{{{\text{SC}}\dot{V}{\text{O}}_{2} }}\) was identified as the point at which \(\tau \dot{V}{\text{O}}_{2}\) consistently deviates from a previously ‘flat’ profile, and the demonstration of a local threshold in the χ2 value (Rossiter et al. 2001). This method allows the fitting of Eq. (1) to the primary component of the response isolated from the slow component, thus avoiding the possibility of arbitrarily parameterizing the slow component. The amplitude of the \(\dot{V}{\text{O}}_{2}\) slow component was determined by calculating the difference between the end-exercise \(\dot{V}{\text{O}}_{2}\) (i.e. mean \(\dot{V}{\text{O}}_{2}\) over final 30 s of exercise) and (\(A\dot{V}{\text{O}}_{2} + \dot{V}{\text{O}}_{{2{\text{b}}}}\)). In instances where exercise duration was too short to allow the slow component to be discerned the \(\dot{V}{\text{O}}_{2}\) response was modelled using Eq. (1) to the end of exercise and the slow component was assigned a value of 0.
Heart rate kinetics were modelled for each exercise transition using a monoexponential function (Eq. 2) with the response constrained to the start of exercise (at t = 0; i.e., with no time delay):
where HRb is the mean HR measured over the final 30 s of baseline cycling, and AHR and τHR are the amplitude and the time constant of the response, respectively.
Statistics
Based on previous knowledge of a meaningful change in \(\tau \dot{V}{\text{O}}_{2}\) during intervention studies (5 s), and a common standard deviation of 4.3 s (Benson et al. 2017), the necessary calculated sample size was 12. Differences in the cardiorespiratory variables between conditions were determined with two-tailed, paired-samples t tests (GraphPad, Prism, USA). Data are presented as means ± SD. Statistical significance was accepted when P < 0.05.
Results
Peak \(\dot{V}{\text{O}}_{2}\) was 2.45 ± 0.61 l min−1 (28.1 ± 5.7 ml kg−1 min−1), with the mean GET occurring at 1.51 ± 0.46 l min−1 (108 ± 39 W). The peak work rate attained from the incremental test was 207 ± 49 W and the work rates calculated to require 80% of the GET and 60% ∆ were 87 ± 29 W and 166 ± 40 W, respectively.
Heart rate kinetics, blood lactate profiles and blood pressure
There were no differences in the primary τHR between PL and CF for moderate- or severe-intensity bouts (P = 0.219 and 0.956, respectively, Table 1). Despite significant changes in blood lactate concentrations at Tlim compared to baseline (P < 0.05; Table 1), there were no significant differences in blood (lactate) from pre- to post-exercise between conditions during moderate- and severe-intensity exercise (see Table 1). Overall, there were no differences between resting systolic (PL 128 ± 12 mmHg vs. CF 127 ± 12 mmHg, P = 0.66) or diastolic (PL 78 ± 7 mmHg vs. 78 ± 7 mmHg, P = 0.75) blood pressure following either PL or CF administration.
\(\dot{V}{\text{O}}_{2}\) kinetics and exercise tolerance
The \(\dot{V}{\text{O}}_{2}\) kinetic parameters for moderate-intensity exercise are presented in Table 2, and the \(\dot{V}{\text{O}}_{2}\) response of a representative participant to moderate-intensity exercise is shown in Fig. 3. Compared with PL, \(\tau \dot{V}{\text{O}}_{2}\) was smaller during moderate-intensity exercise following CF supplementation (PL 40 ± 12 s vs. CF 34 ± 9 s, P = 0.019). However, there were no differences in \(\dot{V}{\text{O}}_{{2{\text{b}}}}\) (P = 0.175), \(A\dot{V}{\text{O}}_{2}\) (P = 0.263), \({\text{TD}}\dot{V}{\text{O}}_{2}\) (P = 0.961) or end exercise \(\dot{V}{\text{O}}_{2}\) (P = 0.565) between PL and CF.
Pulmonary \(\dot{V}{\text{O}}_{2}\) and best-fit modelled responses of a representative participant to moderate-intensity exercise following PL (solid black circles) and CF (clear circles) supplementation. \(\tau \dot{V}{\text{O}}_{2}\) values are displayed for each transition, with the solid grey lines representing the modelled fits
The pulmonary \(\dot{V}{\text{O}}_{2}\) response to severe-intensity exercise for a representative participant is shown in Fig. 4a and group mean responses are shown in Fig. 4b. The associated modelled parameters are presented in Table 2. No impact of CF supplementation on the \(\tau \dot{V}{\text{O}}_{2}\) (P = 0.799) for exercise initiated at 60% ∆ over PL was evident. There were no differences in \(\dot{V}{\text{O}}_{{2{\text{b}}}}\) (P = 0.246), \(A\dot{V}{\text{O}}_{2}\) (P = 0.427), \({\text{TD}}\dot{V}{\text{O}}_{2}\) (P = 0.617), \({\text{SC}}\dot{V}{\text{O}}_{2}\) (P = 0.887) or end exercise \(\dot{V}{\text{O}}_{2}\) (P = 0.954) between conditions. \({\text{TD}}_{{{\text{SC}}\dot{V}{\text{O}}_{2} }}\) was lower following CF vs. PL supplementation (PL 110 ± 15 s vs. CF 95 ± 13 s, P = 0.002). Both end-exercise \(\dot{V}{\text{O}}_{2}\) (P = 0.959) and Tlim (P = 0.480) were not significantly different following PL and CF supplementation during severe-intensity exercise (see Table 2).
Pulmonary \(\dot{V}{\text{O}}_{2}\) and best-fit modelled responses to severe-intensity exercise following PL (solid black circles) and CF (clear black circles) supplementation. Panel a Pulmonary \(\dot{V}{\text{O}}_{2}\) responses of a representative participant displayed with associated \(\tau \dot{V}{\text{O}}_{2}\). Panel b Group mean \(\dot{V}{\text{O}}_{2}\) responses during the rest-to-exercise transition following PL and CF supplementation. Group mean ± SD \(\dot{V}{\text{O}}_{2}\) at limit of exercise tolerance also shown. Solid grey lines represent the modelled fits
Discussion
The purpose of this study was to examine the impact of CFs on pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics during two intensities of cycling exercise in healthy, normotensive middle-aged individuals. Congruent with our hypothesis, the major finding of this study was that 7-days CF supplementation sped pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics during moderate-intensity exercise as demonstrated by a significant reduction in \(\tau \dot{V}{\text{O}}_{2}\). These effects of CFs, however, were not apparent during severe-intensity exercise when compared with a PL. Ultimately, the findings of the present study may have clinical potential in contributing to improved tolerance of daily life activity in middle-aged adults.
Effects of CFs on the physiological responses to moderate-intensity exercise
This study is the first to investigate whether CFs modulate pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics. We show that 7 days CF supplementation significantly reduced the \(\tau \dot{V}{\text{O}}_{2}\) (40 s vs. 34 s) associated with the transition from unloaded to moderate-intensity cycling in middle-aged adults. Notably, the magnitude of change in \(\tau \dot{V}{\text{O}}_{2}\) (~ 6 s) reported is important, as it exceeds the minimum physiologically relevant change of ~ 5 s (Benson et al. 2017). The reduction in \(\tau \dot{V}{\text{O}}_{2}\) observed after CF supplementation in our middle-aged individuals reflects a shift towards values typically observed in younger healthy individuals (Grassi et al. 2009), whereby \(\dot{V}{\text{O}}_{2}\) kinetics are not limited by O2 delivery per se (Poole and Jones 2012). Theoretically, a lowered \(\tau \dot{V}{\text{O}}_{2}\) would reduce the O2 deficit incurred during the exercise transition, thereby causing less perturbations to the intracellular milieu (i.e., ∆ phosphocreatine, ADP, H+, inorganic phosphate, glycogen) and enhancing exercise tolerance (Grassi et al. 2011; Goulding et al. 2017, 2018). Therefore, our data suggest CFs may lower the O2 deficit incurred during moderate-intensity activity by negating age-associated impairments to pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics.
Since the purpose of the study was to examine the impact of CFs on \(\dot{V}{\text{O}}_{2}\) kinetics, our data raise the question about the potential underlying mechanisms contributing to the lowered \(\tau \dot{V}{\text{O}}_{2}\) with CF supplementation. It is acknowledged \(\tau \dot{V}{\text{O}}_{2}\) is sensitive to manipulations in O2 delivery (DeLorey et al. 2004b; Gurd et al. 2009), and, that the slowing of \(\dot{V}{\text{O}}_{2}\) kinetics with advancing age occurs at least partly as a consequence of lowered O2 availability in oxidative skeletal muscle (DeLorey et al. 2004a; Musch et al. 2004; Behnke and Delp 2010). Given that CFs exert potent NO-dependent vasodilatory effects (Schroeter et al. 2006; Cifuentes-Gomez et al. 2015; Decroix et al. 2018a), CF supplementation may have sped \(\dot{V}{\text{O}}_{2}\) kinetics by augmenting muscle blood flow and O2 availability. Although, it is important to acknowledge CFs can alter indices of mitochondrial biogenesis and function (Taub et al. 2012; Kopustinskiene et al. 2015), as well as lower markers of oxidative stress (Ahmed et al. 2020). Together these factors may also influence \(\dot{V}{\text{O}}_{2}\) responses to exercise by augmenting the capacity for O2 utilsisation and delivery. Clearly, futher work is required to determine the mechanisms by which CFs may regulate blood flow and changes in \(\dot{V}{\text{O}}_{2}\) kinetics.
In spite of differences in the kinetics of \(\dot{V}{\text{O}}_{2}\), no changes in the O2 cost of moderate-intensity exercise were observed after CF supplementation. Similarly, Patel et al. (2015) demonstrated no significant reduction in \(\dot{V}{\text{O}}_{2}\) during twenty minutes of moderate-intensity cycling after 14 days dark chocolate supplementation (Patel et al. 2015). Together these findings contrast those published employing alternate dietary means of augmenting NO bioavailability, such as dietary nitrate, which reduces the O2 cost of moderate-intensity activity (Larsen et al. 2007; Bailey et al. 2009; Vanhatalo et al. 2010; Lansley et al. 2011). Such discrepancies may be explained by recent evidence linking dietary nitrate to improved contractile function (Bailey et al. 2019), an effect that has not been reported with CF supplementation. Possibly, the mechanisms by which CFs impact physiological responses to exercise relate to muscle O2 delivery rather than contractile function. Given that we did not measure NO or redox biomarkers, it is not clear to what extent CFs sped phase II \(\dot{V}{\text{O}}_{2}\) kinetics through processes associated with reactive O2 and nitrogen species. Additional research will help delineate CFs mode of action in the context of exercise.
Effects of CFs on the physiological responses to severe-intensity exercise
In contrast to our observations during moderate-intensity exercise, acute CF supplementation had no measurable impact on pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics during severe-intensity cycling. For instance, the \(\tau \dot{V}{\text{O}}_{2}\) of the phase II response was similar between PL and CF (27 s vs. 28 s, respectively). The kinetics of \(\dot{V}{\text{O}}_{2}\) are considered to be an important determinant of exercise tolerance (Whipp and Ward 1992; Grassi et al. 2011). In line with this principle, we observed no effect of CF supplementation on Tlim during severe-intensity exercise. Whilst no previous studies have examined the impact of CF supplementation on \(\dot{V}{\text{O}}_{2}\) kinetics in the severe-intensity exercise domain, a number have studied their effects on exercise performance. Our findings corroborate these data showing no beneficial impact of acute or sub-chronic CF supplementation on time-trial or time-to-exhaustion performance in healthy male adults (Allgrove et al. 2011; Davison et al. 2012; Peschek et al. 2013; Stellingwerff et al. 2014; Decroix et al. 2018b).
Our data demonstrate divergent effects of CFs on \(\dot{V}{\text{O}}_{2}\) kinetics between moderate- and severe-intensity exercise domains. Given that the pattern of muscle-fibre activation within moderate- and severe-intensity exercise domains differs (type I and type II predominant, respectively) (Krustrup et al. 2004), future studies should investigate a potential muscle fibre-type dependency of CF supplementation on the physiological responses to exercise. Another potential explanation for the differences between exercise intensity domains presented herein relates to the dose of CFs administered. Recent published evidence suggests that the 400 mg CF prescribed is the minimum dose necessary to exert beneficial effects during exercise (Decroix et al. 2018a). Therefore, the dose used in the present study may not have been high enough to raise blood flow sufficiently to detect a measurable effect upon \(\dot{V}{\text{O}}_{2}\) kinetics during severe-intensity exercise. In addition, CFs had no beneficial impact on resting systolic or diastolic blood pressure over PL, which may be attributable to insufficient dosage and the normotensive population studied (Hooper et al. 2012). Besides, another limitation of the study is that only a single bout of severe-intensity exercise was conducted. As we were unable to feasibly include additional visits for testing, we could not carry out multiple severe-intensity bouts to enhance the signal-to-noise ratio of these \(\dot{V}{\text{O}}_{2}\) responses and potentially detect differences between conditions. Finally, it is possible that prior exercise, through its effects on (muscle) perfusion, sped the phase II \(\dot{V}{\text{O}}_{2}\) kinetic response during severe-intensity exercise in the population studied (Scheuermann et al. 2002).
Conclusion
In the present study, seven days supplementation with a flavanol-rich cocoa-extract resulted in a reduced \(\tau \dot{V}{\text{O}}_{2}\) during moderate-, but not severe-intensity exercise in normotensive, middle-aged adults. Whilst the O2 cost of exercise was similar between CF and PL conditions, the phase II \(\dot{V}{\text{O}}_{2}\) kinetics were sped at the onset of moderate-intensity exercise after acute CF intake. Such effects on phase II \(\dot{V}{\text{O}}_{2}\) kinetics were not found during severe-intensity exercise with CF. Therefore, CF supplementation may reduce the metabolic perturbations associated with moderate-intensity exercise in middle-aged adults through speeding phase II \(\dot{V}{\text{O}}_{2}\) kinetics.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AHR:
-
Amplitude of the fundamental heart rate response
- \(A\dot{V}{\text{O}}_{2}\) :
-
Amplitude of the phase II oxygen uptake response
- CF:
-
Cocoa flavanol
- GET:
-
Gas exchange threshold
- HR:
-
Heart rate
- HRb :
-
Baseline heart rate
- τHR:
-
Time constant of the fundamental heart rate response
- NO:
-
Nitric oxide
- O2 :
-
Oxygen
- PL:
-
Placebo
- \({\text{SC}}\dot{V}{\text{O}}\) :
-
Magnitude of the slow component
- \(\tau \dot{V}{\text{O}}_{2}\) :
-
Time constant of the phase II response
- \({\text{TD}}_{{{\text{SC}}\dot{V}{\text{O}}_{2} }}\) :
-
Time delay of the \(\dot{V}{\text{O}}_{2}\) slow component
- \({\text{TD}}\dot{V}{\text{O}}_{2}\) :
-
Time delay of the phase II response
- Tlim :
-
Limit of exercise tolerance
- \(\dot{V}{\text{O}}_{2}\) :
-
Oxygen uptake
- \(\dot{V}{\text{O}}_{{2{\text{b}}}}\) :
-
Baseline oxygen uptake
References
Ahmed S, Ahmed N, Rungatscher A et al (2020) Cocoa flavonoids reduce inflammation and oxidative stress in a myocardial ischemia-reperfusion experimental model. Antioxidants. https://doi.org/10.3390/antiox9020167
Allgrove J, Farrell E, Gleeson M et al (2011) Regular dark chocolate consumption’s reduction of oxidative stress and increase of free-fatty-acid mobilization in response to prolonged cycling. Int J Sport Nutr Exerc Metab 21(2):113–123. https://doi.org/10.1123/ijsnem.21.2.113
Bailey SJ, Winyard P, Vanhatalo A et al (2009) Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol 107(4):1144–1155. https://doi.org/10.1152/japplphysiol.00722.2009
Bailey SJ, Varnham RL, DiMenna FJ et al (2015) Inorganic nitrate supplementation improves muscle oxygenation, O2 uptake kinetics, and exercise tolerance at high but not low pedal rates. J Appl Physiol 118(11):1396–1405. https://doi.org/10.1152/japplphysiol.01141.2014
Bailey SJ, Gandra PG, Jones AM et al (2019) Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological PO2. J Physiol 597(22):5429–5443. https://doi.org/10.1113/JP278494
Beaver WL, Lamarra N, Wasserman K (1981) Breath-by-breath measurement of true alveolar gas exchange. J Appl Physiol Respir Environ Exerc Physiol 51(6):1662–1675. https://doi.org/10.1152/jappl.1981.51.6.1662
Beaver WL, Wasserman K, Whipp BJ (2016) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 121(6):2020–2027
Behnke BJ, Delp MD (2010) Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol 108(1):14–20. https://doi.org/10.1152/japplphysiol.00970.2009
Benson AP, Bowen TS, Ferguson C et al (2017) Data collection, handling, and fitting strategies to optimize accuracy and precision of oxygen uptake kinetics estimation from breath-by-breath measurements. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00988.2016
Berry NM, Davison K, Coates AM et al (2010) Impact of cocoa flavanol consumption on blood pressure responsiveness to exercise. Br J Nutr 103(10):1480–1484. https://doi.org/10.1017/S0007114509993382
Burnley M, Jones AM, Carter H, Doust JH (2000) Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol. https://doi.org/10.1152/jappl.2000.89.4.1387
Cifuentes-Gomez T, Rodriguez-Mateos A, Gonzalez-Salvador I et al (2015) Factors affecting the absorption, metabolism, and excretion of cocoa flavanols in humans. J Agric Food Chem 63(35):7615–7623. https://doi.org/10.1021/acs.jafc.5b00443
Cunningham DA, Paterson DH (1994) Exercise on-transient gas exchange kinetics are slowed as a function of age. Med Sci Sports Exerc. https://doi.org/10.1249/00005768-199404000-00007
Davison K, Coates AM, Buckley JD, Howe PRC (2008) Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes 32(8):1289–1296. https://doi.org/10.1038/ijo.2008.66
Davison G, Callister R, Williamson G et al (2012) The effect of acute pre-exercise dark chocolate consumption on plasma antioxidant status, oxidative stress and immunoendocrine responses to prolonged exercise. Eur J Nutr 51(1):69–79. https://doi.org/10.1007/s00394-011-0193-4
Decroix L, Soares DD, Meeusen R et al (2018a) Cocoa flavanol supplementation and exercise: a systematic review. Sport Med 48:867–892. https://doi.org/10.1007/s40279-017-0849-1
Decroix L, Tonoli C, Lespagnol E et al (2018b) One-week cocoa flavanol intake increases prefrontal cortex oxygenation at rest and during moderate-intensity exercise in normoxia and hypoxia. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00055.2018
DeLorey DS, Kowalchuk JM, Paterson DH (2004a) Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J Appl Physiol 97(1):165–172. https://doi.org/10.1152/japplphysiol.01179.2003
DeLorey DS, Kowalchuk JM, Paterson DH (2004b) Effects of prior heavy-intensity exercise on pulmonary O2 uptake and muscle deoxygenation kinetics in young and older adult humans. J Appl Physiol. https://doi.org/10.1152/japplphysiol.01280.2003
Dumanoir GR, Delorey DS, Kowalchuk JM, Paterson DH (2010) Differences in exercise limb blood flow and muscle deoxygenation with age: contributions to O2 uptake kinetics. Eur J Appl Physiol 110(4):739–751. https://doi.org/10.1007/s00421-010-1546-z
George MA, McLay KM, Doyle-Baker PK et al (2018) Fitness level and not aging per se, determines the oxygen uptake kinetics response. Front Physiol. https://doi.org/10.3389/fphys.2018.00277
Goulding RP, Roche DM, Marwood S (2017) Prior exercise speeds pulmonary oxygen uptake kinetics and increases critical power during supine but not upright cycling. Exp Physiol 102(9):1158–1176. https://doi.org/10.1113/EP086304
Goulding RP, Roche DM, Marwood S (2018) “Work-to-Work” exercise slows pulmonary oxygen uptake kinetics, decreases critical power, and increases W’during supine cycling. Physiol Rep 6(21):e13916
Grassi B, Porcelli S, Marzorati M et al (2009) Metabolic myopathies: functional evaluation by analysis of oxygen uptake kinetics. Med Sci Sports Exerc 41(12):2120–2127. https://doi.org/10.1249/MSS.0b013e3181aae96b
Grassi B, Porcelli S, Salvadego D, Zoladz JA (2011) Slow V̇O2 kinetics during moderate-intensity exercise as markers of lower metabolic stability and lower exercise tolerance. Eur J Appl Physiol 111(3):345–355. https://doi.org/10.1007/s00421-010-1609-1
Gurd BJ, Peters SJ, Heigenhauser GJF et al (2009) Prior heavy exercise elevates pyruvate dehydrogenase activity and muscle oxygenation and speeds O2 uptake kinetics during moderate exercise in older adults. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.90848.2008
Hooper L, Kay C, Abdelhamid A et al (2012) Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 95(3):740–751. https://doi.org/10.3945/ajcn.111.023457
Kopustinskiene DM, Savickas A, Vetchý D et al (2015) Direct effects of (-)-Epicatechin and procyanidin B2 on the respiration of rat heart mitochondria. Biomed Res Int. https://doi.org/10.1155/2015/232836
Krustrup P, Söderlund K, Mohr M, Bangsbo J (2004) The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch Eur J Physiol. https://doi.org/10.1007/s00424-003-1203-z
Lamarra N, Whipp BJ, Ward SA, Wasserman K (1987) Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 62(5):2003–2012
Lansley KE, Winyard PG, Fulford J et al (2011) Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol 110(3):591–600. https://doi.org/10.1152/japplphysiol.01070.2010
Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B (2007) Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol 191(1):59–66. https://doi.org/10.1111/j.1748-1716.2007.01713.x
McNarry MA, Kingsley MIC, Lewis MJ (2012) Influence of exercise intensity on pulmonary oxygen uptake kinetics in young and late middle-aged adults. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00203.2012
Muller-Delp JM, Spier SA, Ramsey MW, Delp MD (2002) Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Hear Circ Physiol 283(4):H1662
Murias JM, Paterson DH (2015) Slower VO2 kinetics in older individuals: is it inevitable? Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0000000000000686
Murias JM, Kowalchuk JM, Peterson DH (2010) Speeding of VO2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization. J Appl Physiol 108(4):913–922. https://doi.org/10.1152/japplphysiol.01355.2009
Musch TI, Eklund KE, Hageman KS, Poole DC (2004) Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96(1):81–88. https://doi.org/10.1152/japplphysiol.00729.2003
Patel RK, Brouner J, Spendiff O (2015) Dark chocolate supplementation reduces the oxygen cost of moderate intensity cycling. J Int Soc Sports Nutr. https://doi.org/10.1186/s12970-015-0106-7
Peschek K, Pritchett R, Bergman E, Pritchett K (2013) The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients 6(1):50–62. https://doi.org/10.3390/nu6010050
Poole DC, Jones AM (2012) Oxygen uptake kinetics. Compr Physiol 2(2):933–996. https://doi.org/10.1002/cphy.c100072
Poole DC, Barstow TJ, Mcdonough P, Jones AM (2008) Control of oxygen uptake during exercise. Med Sci Sports Exerc 40:462–474. https://doi.org/10.1249/MSS.0b013e31815ef29b
Rossiter HB, Ward SA, Kowalchuk JM et al (2001) Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol 537(1):291–303. https://doi.org/10.1111/j.1469-7793.2001.0291k.x
Scheuermann BW, Bell C, Paterson DH et al (2002) Oxygen uptake kinetics for moderate exercise are speeded in older humans by prior heavy exercise. J Appl Physiol. https://doi.org/10.1152/japplphysiol.00186.2001
Schreuder THA, Green DJ, Nyakayiru J et al (2015) Time-course of vascular adaptations during 8 weeks of exercise training in subjects with type 2 diabetes and middle-aged controls. Eur J Appl Physiol. https://doi.org/10.1007/s00421-014-3006-7
Schroeter H, Heiss C, Balzer J et al (2006) (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103(4):1024–1029. https://doi.org/10.1073/pnas.0510168103
Sindler AL, Delp MD, Reyes R et al (2009) Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587(15):3885–3897. https://doi.org/10.1113/jphysiol.2009.172221
Spencer MD, Murias JM, Lamb HP et al (2011) Are the parameters of VO2, heart rate and muscle deoxygenation kinetics affected by serial moderate-intensity exercise transitions in a single day? Eur J Appl Physiol. https://doi.org/10.1007/s00421-010-1653-x
Spier SA, Delp MD, Meininger CJ et al (2004) Effects of ageing and exercise training on endothelium-dependent vasodilation and structure of rat skeletal muscle arterioles. J Physiol 556(3):947–958. https://doi.org/10.1113/jphysiol.2003.060301
Stellingwerff T, Godin JP, Chou CJ et al (2014) The effect of acute dark chocolate consumption on carbohydrate metabolism and performance during rest and exercise. Appl Physiol Nutr Metab 39(2):173–182. https://doi.org/10.1139/apnm-2013-0152
Taub PR, Ramirez-Sanchez I, Ciaraldi TP et al (2012) Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: effects of epicatechin rich cocoa. Clin Transl Sci. https://doi.org/10.1111/j.1752-8062.2011.00357.x
Vanhatalo A, Bailey SJ, Blackwell JR et al (2010) Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00206.2010
Whipp BJ, Rossiter HB (2013) The kinetics of oxygen uptake: physiological inferences from the parameters. In: Jones AM, Poole DC (eds) Oxygen uptake kinetics in sport, exercise and medicine. Routledge, London, UK, pp 1–406
Whipp BJ, Ward SA (1992) Pulmonary gas exchange dynamics and the tolerance to muscular exercise: effects of fitness and training. Ann Physiol Anthropol 11:207–214. https://doi.org/10.2114/ahs1983.11.207
Woodman CR, Price EM, Laughlin MH (2002) Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol 93(5):1685–1690. https://doi.org/10.1152/japplphysiol.00461.2002
Funding
The study was supported by funding received from the Biotechnology and Biological Sciences Research Council (BBSRC) and Unilever R & D Vlaardingen, The Netherlands.
Author information
Authors and Affiliations
Contributions
DGS, CS, HJ and DHJT conceived and designed the project, DGS collected and analysed the data, DGS, HJ, CS, RD, SM and DHJT interpreted the data. DGS drafted the manuscript and all authors revised it critically. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All people designated as authors qualify for authorship, and all those who qualify for authorship are listed. DHJT is the guarantor for the work and/or conduct of the study, had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of the data analysis, and controlled the decision to publish.
Corresponding author
Ethics declarations
Conflict of interest
Daniel G. Sadler, Claire E. Stewart, Helen Jones, Simon Marwood and Dick H. J. Thijssen had no conflict of interest associated with this manuscript. Richard Draijer is employed by Unilever R & D Vlaardingen, The Netherlands. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethics approval
All procedures conformed to the Declaration of Helsinki and were approved by Liverpool John Moores University Research Ethics Committee (approval reference number: 18/SPS/014).
Consent to participate
All participants volunteered and gave written informed consent to participate in the study.
Consent for publication
All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Additional information
Communicated by Michalis G Nikolaidis .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadler, D.G., Draijer, R., Stewart, C.E. et al. Cocoa-flavanols enhance moderate-intensity pulmonary \(\dot{V}{\text{O}}_{2}\) kinetics but not exercise tolerance in sedentary middle-aged adults. Eur J Appl Physiol 121, 2285–2294 (2021). https://doi.org/10.1007/s00421-021-04682-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-021-04682-9