

Ophthalmic and
Leaders in Advanced Aseptic Pharmaceutical Manufacturing
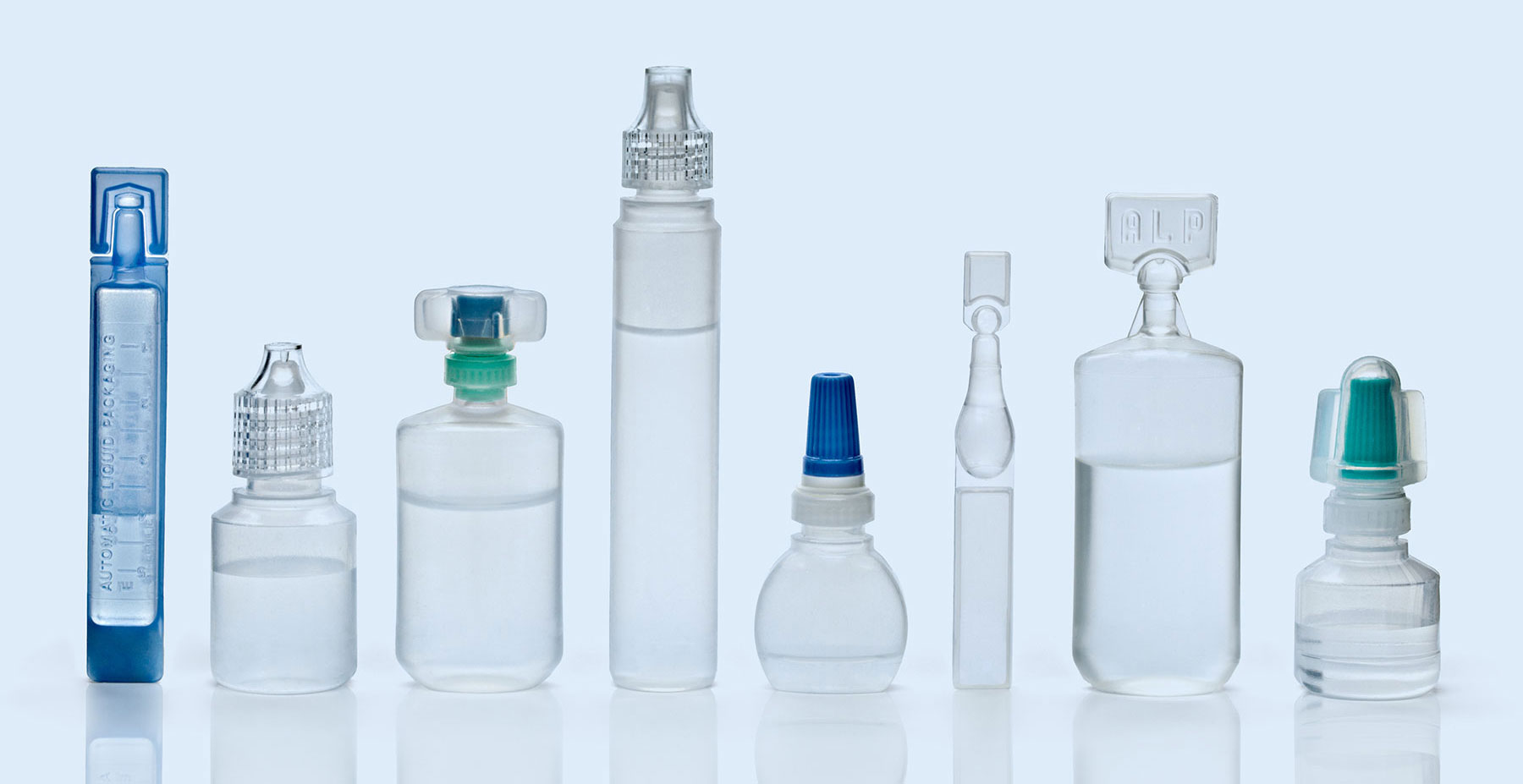
Delivering Sterile Products Through Blow-Fill-Seal Technology
Woodstock Sterile Solutions is a leading global provider of best-in-class sterile development and manufacturing solutions — with a decades-long track record of helping healthcare companies advance molecules through clinical development into full-scale commercial production through blow-fill-seal technology.
Woodstock Sterile Solutions is one of the leading contract development and manufacturing (CDMO) companies for aseptic pharmaceutical manufacturing of sterile liquid formulations. This ensures your products will be on target and on time.
Aseptic Manufacturing Process
Advanced aseptic blow-fill-seal (BFS) technology is used extensively for liquid filling of primary containers for solutions, suspensions, and emulsions, but organizations are hesitant to use blow-fill-seal technology for biologics and large molecule formulations due to the products’ thermal sensitivity and the heat generated during the blow-fill-seal technology process.
Woodstock Sterile Solutions has extensive experience and proven history of manufacturing biologics in blow-fill-seal with our patented processing to minimize and control the potential heat impact on the product. Dr. Waiken Wong, our Manager of Development Engineering, explains the technology and discusses the other advantages of using blow-fill-seal technology in his webinar, Blow-Fill-Seal: Advanced Aseptic Manufacturing for Temperature-Sensitive Drug Products. To view the webinar, contact us.
Blow-Fill-Seal Technology
Why Woodstock?
Expertise in Complex
Specialized in handling complex solutions, suspensions, and emulsions.
Flexible BFS Technology Options
Cost-efficient, standard bottle sizes or custom-designed containers.
Capacity & Scale
From clinical development to large-scale commercial projects.
Manufacturing Know-How
Insertion Technology
Unique capability for aseptic insertion of components such as tip/cap assemblies.
Innovation
Our commitment to innovation reduces development time for our customers.
Faster to Market
API Stewardship
Regulatory Expertise
Global Quality
At Woodstock Sterile Solutions, your first delivery system can be your final delivery system — increasing speed to market and streamlining the scale-up process.

Trusted, Efficient Development Capabilities
Broad Registration, Accreditations, and Certifications
- Registered for drug, device, and food manufacturing with the U.S. Food and Drug Administration (FDA)
- State license for wholesale drug manufacturing and distribution licenses
- Certifications through EU-competent authorities
- Manufacturing accreditations for Japan, Australia, Canada, and Brazil
- Potent material handling Safebridge 1-4
- ISO 13485:2016 certification








