Our Story
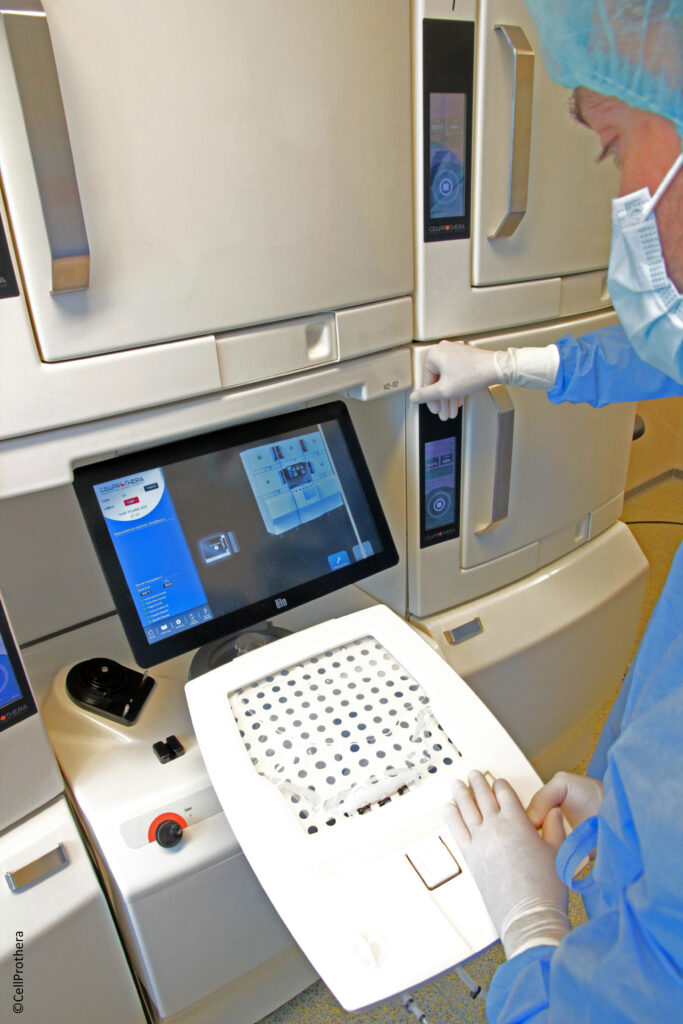
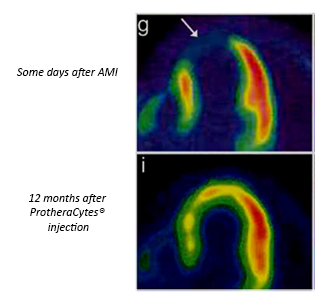
In 2002, a successful Proof of Concept clinical study led by Pr Philippe HENON – Founder and Director of IRHT Mulhouse - showing structural and functional regeneration of myocardial tissue as well as reperfusion of an infarcted zone after a recent Acute Myocardial Infarct (AMI) was performed and included 7 myocardial infarction patients with a very poor short-term prognosis. Based on the objective of building a robust and automated bioprocessing technology aimed at producing large numbers of purified stem cells, CellProthera has developed a proprietary technology platform made of an automated expansion device called StemXpand® and its disposable kit StemPack®. The final cell product ProtheraCytes® is made of CD34+ stem cells which have the potential for regeneration of various damaged tissues. ProtheraCytes® is registered as an ATMP – Advanced Therapy Medicinal Product – within the classification of Tissue Engineered product by the European Medicines Agency. Our most advanced program is the regeneration of damaged heart tissue after severe AMI to prevent Heart Failure (HF). It is an unmet medical need suffered by over a million patients per year in the US, Europe and Japan. ...